ACTIVITY: SOLUTION CONCENTRATION VS. CONDUCTIVITY
Here is your goal for this lesson:
Gr...

Chemistry, 16.04.2021 19:30 tanya44737
ACTIVITY: SOLUTION CONCENTRATION VS. CONDUCTIVITY
Here is your goal for this lesson:
Graph experimental data and interpret results for peer review
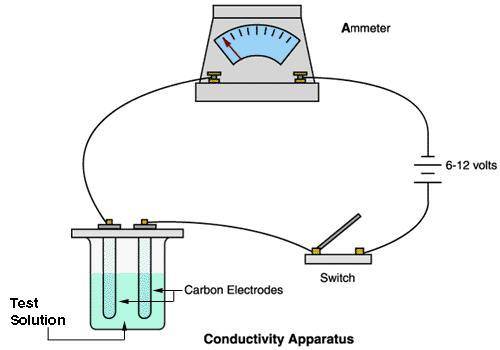
A chemistry student carried out an experiment with a conducting apparatus (ammeter) similar to the one below. This ammeter measures in milliamperes (mA). The following data was taken.
Solution Reading
0.1 M H2SO4 150 mA
0.1 M Ba(OH)2 150 mA
To 30 mL of the Ba(OH)2 solution, 10 mL portions of H2SO4 were added until a total of 50 mL of H2SO4 were used. The following results were recorded.
DATA TABLE
Total H2SO4 Added Meter Reading Observations
0 mL 150 mA Ba(OH)2 and H2SO4 clear, colorless
10 mL 65 mA milky white precipitate forms
20 mL 31 mA more precipitate forms
30 mL 0 mA precipitate heavy and settles
40 mL 29 mA no added precipitate seen to form
50 mL 62 mA no change seen
Questions
1. Did you plot the data?
yes
no
2. Did you label your graph axes?
no
yes
3. Did you give your graph a title?
no
yes
4. Does the Ba(OH)2 solution contain ions?
yes
no
5. Does the H2SO4 solution contain ions?
yes
no
6. Explain the data.
Is there any evidence that a reaction has occurred?
7. Does the conductivity increase or decrease?
8. Does the number of ions in solution increase, decrease, or remain constant?
9. What is the indicator of the number of ions in solution?
10. How does this evidence indicate that the reaction has occurred between ions?
11. The Ba(OH)2 dissociates as Ba+2 + 2 OH-. H2SO4 dissociates as 2 H+ + SO4-2.
Write a balanced equation for this reaction.
12. When the conductivity is at a minimum, what must be true about the amount of Ba(OH)2?
13. Why does it not conduct at this low point?
14. Why does it conduct more before and after this minimum point?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1
You know the right answer?
Questions



Computers and Technology, 01.08.2019 06:40

History, 01.08.2019 06:40

Mathematics, 01.08.2019 06:40





English, 01.08.2019 06:40

Mathematics, 01.08.2019 06:40



Mathematics, 01.08.2019 06:40

History, 01.08.2019 06:40



Social Studies, 01.08.2019 06:40

Social Studies, 01.08.2019 06:40



