
Chemistry, 06.10.2019 00:30 twistedhyperboles
The first step in the reaction of alka-seltzer with stomach acid consists of one mole of sodium bicarbonate (nahco3) reacting with one mole of hydrochloric acid (hcl) to produce one mole of carbonic acid (h2co3), and one mole of sodium chloride (nacl). using this chemical stoichiometry, determine the number of moles of carbonic acid that can be produced from 5 mol of nahco3 and 8 mol of hcl.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3
You know the right answer?
The first step in the reaction of alka-seltzer with stomach acid consists of one mole of sodium bica...
Questions


English, 04.03.2021 20:00




Mathematics, 04.03.2021 20:00


Mathematics, 04.03.2021 20:00


English, 04.03.2021 20:00

History, 04.03.2021 20:00


Chemistry, 04.03.2021 20:00




Mathematics, 04.03.2021 20:00

Mathematics, 04.03.2021 20:00


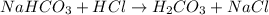
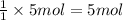 of HCl
of HCl


