
Chemistry, 06.10.2019 10:00 kaylaamberd
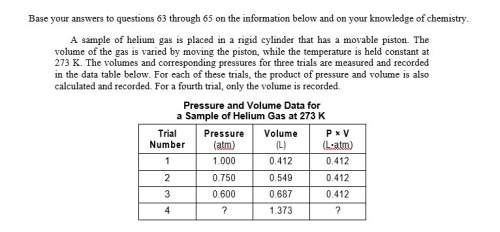
Asample of helium gas is placed in a rigid cylinder that has a movable piston. the volume of the gas is varied by moving the piston, while the temperature is held constant at 273 k. the volumes and corresponding pressures for three trials are measured and recorded in the data table below. for each of these trials, the product of pressure and volume is also calculated and recorded. for a fourth trial, only the volume is recorded.
determine the pressure of the helium gas in trial 4. [1]

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2
You know the right answer?
Asample of helium gas is placed in a rigid cylinder that has a movable piston. the volume of the gas...
Questions


History, 03.04.2021 03:00


Mathematics, 03.04.2021 03:00



Physics, 03.04.2021 03:00





Mathematics, 03.04.2021 03:00



Mathematics, 03.04.2021 03:00





Mathematics, 03.04.2021 03:00



