
Chemistry, 24.12.2019 06:31 fernandancon1872
What is the effect of adding more co2 to the following equilibrium reaction? co2 + h2o h2co3
a. more h2co3 is produced.
b. more h2o is produced.
c. the equilibrium is pushed in the direction of reactants.
d. no change
also,
which of the following is the correct expression for the rate of the following reaction? note: all species are gaseous.2so3 < --> 2so2 + o2a. [so3]2 / ([so2]2 x [o2])
b. [so2]2 x [o2] / [so3]
c. [so2]2 x [o2] / [so3]2
d. [so2] x [o2] / [so3]

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3


Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1
You know the right answer?
What is the effect of adding more co2 to the following equilibrium reaction? co2 + h2o h2co3
<...
<...
Questions


Mathematics, 28.07.2019 13:10

Geography, 28.07.2019 13:10

Mathematics, 28.07.2019 13:10



Biology, 28.07.2019 13:10

Chemistry, 28.07.2019 13:10

Physics, 28.07.2019 13:10

Chemistry, 28.07.2019 13:10

Mathematics, 28.07.2019 13:10


Mathematics, 28.07.2019 13:10


History, 28.07.2019 13:10


Mathematics, 28.07.2019 13:10

Mathematics, 28.07.2019 13:10


Mathematics, 28.07.2019 13:10

![K=\frac{[SO_2]^2\times[O_2]}{[SO_3]^2}](/tpl/images/0431/4779/57391.png)
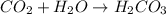
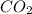 is increased, that is the reactant is increased, so according to the Le-Chatlier's principle, the equilibrium will shift in the direction where decrease of concentration of
is increased, that is the reactant is increased, so according to the Le-Chatlier's principle, the equilibrium will shift in the direction where decrease of concentration of 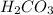 will be formed.
will be formed.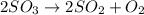
![K=\frac{[SO_2]^2\times [O_2]}{[SO_3]^2}](/tpl/images/0431/4779/8f21b.png)


