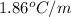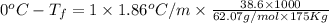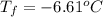Chemistry, 10.10.2019 19:30 jalenshayewilliams
Ethylene glycol, c2h6o2, is a nonvolatile substance unable to form ions in water. if 38.6 grams of ethylene glycol is dissolved in 175 grams of water, what is the freezing point of the solution? kf = 1.86°c/m; kb = 0.512°c/m
-6.61°c
1.82°c
6.61°c
-1.82°c

Answers: 3
Another question on Chemistry


Chemistry, 21.06.2019 18:30
In an oxidation-reduction reaction, oxidation is what happens when a reactant
Answers: 1

Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2
You know the right answer?
Ethylene glycol, c2h6o2, is a nonvolatile substance unable to form ions in water. if 38.6 grams of e...
Questions

Mathematics, 15.08.2021 01:00

Mathematics, 15.08.2021 01:00






Mathematics, 15.08.2021 01:00



Mathematics, 15.08.2021 01:00


Chemistry, 15.08.2021 01:00


Mathematics, 15.08.2021 01:00




Mathematics, 15.08.2021 01:00

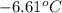
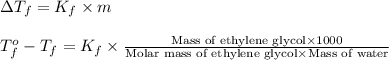
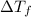 = change in freezing point
= change in freezing point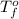 = temperature of pure water =
= temperature of pure water = 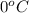
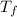 = temperature of solution = ?
= temperature of solution = ?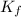 = freezing point constant =
= freezing point constant =