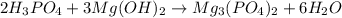Chemistry, 20.01.2020 20:31 brenyasanders5345
An acid (x) reacts with a base (y) to produce mg3(po4)2. what are x and y? x = h3po3; y = mg(oh)2 x = h3po4; y = mg(oh)2 x = h3po4; y = ca(oh)2 x = h3po3; y = ca(oh)2

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1
You know the right answer?
An acid (x) reacts with a base (y) to produce mg3(po4)2. what are x and y? x = h3po3; y = mg(oh)2...
Questions

Chemistry, 06.07.2019 23:30




History, 06.07.2019 23:30



History, 06.07.2019 23:30

Mathematics, 06.07.2019 23:30

History, 06.07.2019 23:30

History, 06.07.2019 23:30


Mathematics, 06.07.2019 23:30

Mathematics, 06.07.2019 23:30



Physics, 06.07.2019 23:30



Biology, 06.07.2019 23:30

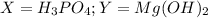

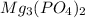 . So, the acid must be phosphoric acid and the base must be magnesium hydroxide.
. So, the acid must be phosphoric acid and the base must be magnesium hydroxide.