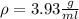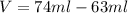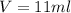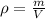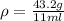Chemistry, 18.09.2019 21:00 miguelelmenor910
The philosopher’s stone weighs 43.2 g is placed in a graduated cylinder containing 63 ml of water. after the stone is added to the cylinder the water rises to 74 ml. what is the density of the stone? express your answer in g/ml.

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 04:00
Which method would be best to separate a mixture of sand and gravel
Answers: 1

Chemistry, 23.06.2019 11:00
The lab procedure involves several factors, listed below some were variable and some were constant. label each factor below v for variable ot c for constant
Answers: 1

Chemistry, 23.06.2019 13:00
How many grams of oxygen gas will react completely with a block of calcium metal that is 3.0 cm by 3.5 cm by 4.2 cm, if the density of calcium is 1.55 g/ml? show all steps of your calculation as well as the final answer.
Answers: 3

Chemistry, 23.06.2019 14:00
[07.06] which of the following chemical reactions is an oxidation-reduction reaction? (2 points) wo3 + 3h2 yields w + 3h2o kno3 + licl yields lino3 + kcl caso4 + 2nacl yields na2so4 + cacl2 mg(no3)2 + 2hbr yields mgbr2 + 2hno3
Answers: 1
You know the right answer?
The philosopher’s stone weighs 43.2 g is placed in a graduated cylinder containing 63 ml of water. a...
Questions


English, 16.07.2019 04:00



Social Studies, 16.07.2019 04:00

Social Studies, 16.07.2019 04:00


Social Studies, 16.07.2019 04:00

English, 16.07.2019 04:00

Biology, 16.07.2019 04:00

Biology, 16.07.2019 04:00

English, 16.07.2019 04:00

English, 16.07.2019 04:00


Mathematics, 16.07.2019 04:00


Mathematics, 16.07.2019 04:00


Biology, 16.07.2019 04:00