
Chemistry, 04.02.2020 05:01 lalala1212
1.) why does carbon form covalent bonds?
it tends to pull 4 electrons from another atom.
it tends to release 4 electrons to another atom.
it will usually bond to multiple atoms which can provide a total of 4 additional electrons.
it has a strong electronegativity.
2.) what type of bond will be formed for atoms that have a +1 or -1 charge?
covalent
ionic
they do not react
elemental
3.) select all that apply.
the relative ionic or covalent character of chemical bonds may be estimated by:
the total number of electrons for each element
the addition of electronegativities
comparison of the associated families to which the elements belong
the difference in electronegativities

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3


Chemistry, 23.06.2019 09:00
A2-kg bowling ball is 1 meter off the ground on a post when it falls. just before it reaches the ground,its traveling 4.4 m/s. assuming that there is no air resistant, which statement is true a. the initial potential energy is less then the final kinetic energy b. the mechanical energy is not conserved c. the mechanical energy is conserved d. the initial potential energy is greater than the final kinetic energy
Answers: 3
You know the right answer?
1.) why does carbon form covalent bonds?
it tends to pull 4 electrons from another atom...
it tends to pull 4 electrons from another atom...
Questions


Spanish, 13.02.2020 23:32






Mathematics, 13.02.2020 23:32


Arts, 13.02.2020 23:32

Computers and Technology, 13.02.2020 23:32



Biology, 13.02.2020 23:32

English, 13.02.2020 23:32

Geography, 13.02.2020 23:32




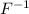 ion and sodium on losing an electron will form a
ion and sodium on losing an electron will form a 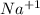 ion.
ion.

