
Chemistry, 21.04.2021 06:20 brunovillarreal6576
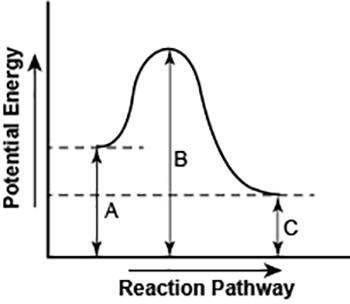
Part 1: Looking at the diagram above, what can you tell me about the type of reaction it is? Is this exothermic or endothermic? How do you know? Make sure you support your answer by using the diagram.
Part 2: Thinking about enthalpy and a change in enthalpy, explain how you could find the total change of enthalpy based on this diagram. Is the enthalpy positive or negative?
Part 3: How could you find the activation energy? Is the activation energy positive or negative?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1


Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
Part 1: Looking at the diagram above, what can you tell me about the type of reaction it is? Is this...
Questions






















