
Chemistry, 23.04.2021 18:30 pulliamdylan
If the temperature of the gas molecules shown below were reduced to 200 K, what pressure would they exert? (Assume constant volume.)
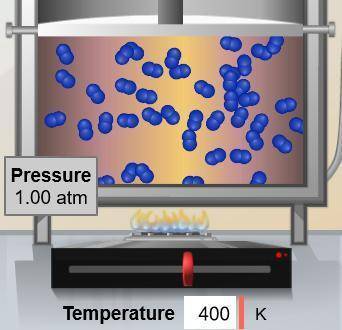

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3


Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3
You know the right answer?
If the temperature of the gas molecules shown below were reduced to 200 K, what pressure would they...
Questions





History, 22.02.2020 02:07




Computers and Technology, 22.02.2020 02:07














