
Chemistry, 24.04.2021 14:00 joannakawata6
A gas at 1.10 atm and 30.0°C fills a flexible container with an initial volume of 2.00 L. If the temperature is raised to 80.0°C and the pressure increased to 2.40 atm, what is the new volume?
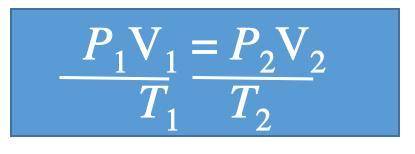

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1
You know the right answer?
A gas at 1.10 atm and 30.0°C fills a flexible container with an initial volume of 2.00 L. If the tem...
Questions


Mathematics, 26.07.2019 02:40






History, 26.07.2019 02:40

Mathematics, 26.07.2019 02:40


Business, 26.07.2019 02:40


Chemistry, 26.07.2019 02:40


Computers and Technology, 26.07.2019 02:40







