
Chemistry, 30.04.2021 02:40 HotWheels162000
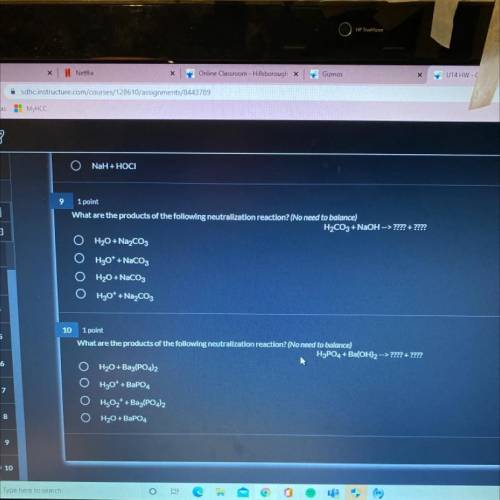
What are the products of the following neutralization reaction? (No need to balance)
H2CO3 + NaOH --> ? ?
A.)H2O + Na2CO3
B.)H30+ + NaCO3
C.)H20 + NaCO3
D.)H30+ + Na2CO3

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Does anyone know a lot about how to: - calculate mass of magnesium metal - calculate the actual yield of magnesium oxide - calculate the theoretical yield of mgo - calculate the percent yield of mgo - determine the percent yield of mgo - determine the average percent yield of mgo i had to do an online lab and its asking these questions but i have no idea where to start or how to be able to find these things. i can post the chart of the data from the lab or if you can tell me exactly how i can find each.
Answers: 3


Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2

You know the right answer?
What are the products of the following neutralization reaction? (No need to balance)
H2CO3 + NaOH...
Questions

Social Studies, 10.09.2020 02:01

Social Studies, 10.09.2020 02:01

Social Studies, 10.09.2020 02:01


Social Studies, 10.09.2020 02:01

Social Studies, 10.09.2020 02:01

English, 10.09.2020 02:01



Mathematics, 10.09.2020 02:01



History, 10.09.2020 02:01


Social Studies, 10.09.2020 02:01

Social Studies, 10.09.2020 02:01

Computers and Technology, 10.09.2020 02:01

Mathematics, 10.09.2020 02:01

History, 10.09.2020 02:01

Mathematics, 10.09.2020 02:01



