
Chemistry, 30.04.2021 03:20 tramqpham25
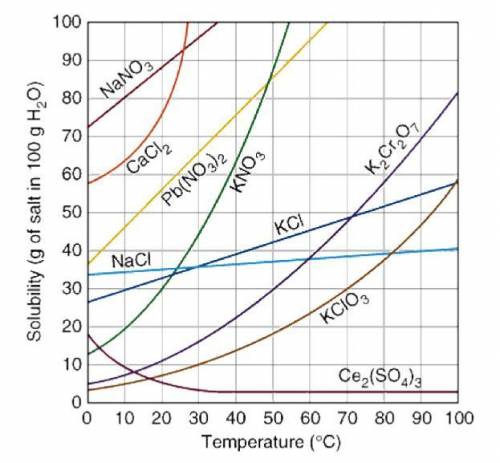
PLEASE SOMEONE HELP ME A mass of 50g of KNO3 is dissolved in 100 g of water at 32°C. The solution is heated to 50°C. How many more grams of potassium nitrate must be added to make the solution saturated? Explain your reasoning.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
You know the right answer?
PLEASE SOMEONE HELP ME
A mass of 50g of KNO3 is dissolved in 100 g of water at 32°C. The solution...
Questions

English, 29.08.2019 19:10

Social Studies, 29.08.2019 19:10















Computers and Technology, 29.08.2019 19:10



Computers and Technology, 29.08.2019 19:10



