
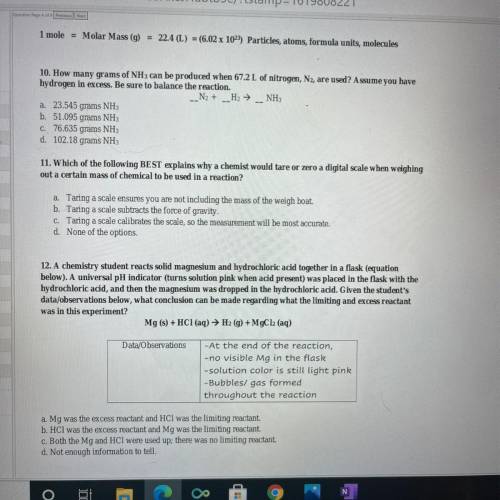
10. How many grams of NH 3 can be produced when 67.2 L of nitrogen, , are used? Assume you have N 2 hydrogen in excess. Be sure to balance the reaction. 12. A chemistry student reacts solid magnesium and hydrochloric acid together in a flask (equation below). A universal pH indicator (turns solution pink when acid present) was placed in the flask with the hydrochloric acid, and then the magnesium was dropped in the hydrochloric acid. Given the student's data/ observations below, what conclusion can be made regarding what the limiting and excess reactant was in this experiment?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3
You know the right answer?
10. How many grams of NH 3 can be produced when 67.2 L of nitrogen, , are used? Assume you have N 2...
Questions

SAT, 16.07.2019 01:00


Mathematics, 16.07.2019 01:00


Mathematics, 16.07.2019 01:00


Health, 16.07.2019 01:00

History, 16.07.2019 01:00





Geography, 16.07.2019 01:00


Mathematics, 16.07.2019 01:00


Mathematics, 16.07.2019 01:00





