
Chemistry, 05.05.2021 08:40 EllaLovesAnime
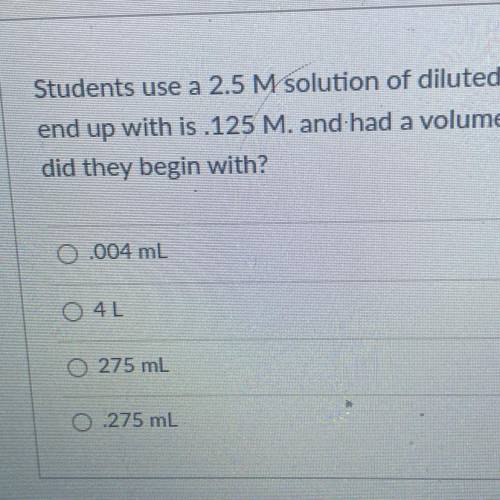
Students use a 2.5 M solution of diluted hydrochloric acid. The Molarity of the solution they
end up with is 125 M. and had a volume of 5500mL. What volume of the concentrated HCI
did they begin with?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
Students use a 2.5 M solution of diluted hydrochloric acid. The Molarity of the solution they
end...
Questions

Computers and Technology, 20.01.2020 23:31

Computers and Technology, 20.01.2020 23:31

Computers and Technology, 20.01.2020 23:31















Computers and Technology, 20.01.2020 23:31




