
Chemistry, 13.05.2021 17:00 SmoothCruzito16
Calculate the mass in grams of water vapor produced when 1.8 L of methane gas undergo complete combustion at a temperature of 281.6°c and a pressure of 1.00 atm. Write the complete balance equation and solve the stoichometry.
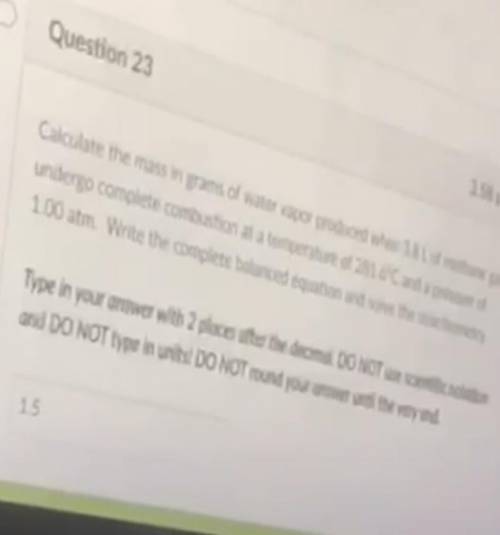

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
Calculate the mass in grams of water vapor produced when 1.8 L of methane gas undergo complete combu...
Questions







Computers and Technology, 07.06.2020 05:00















