
Chemistry, 17.05.2021 09:40 xxtonixwilsonxx
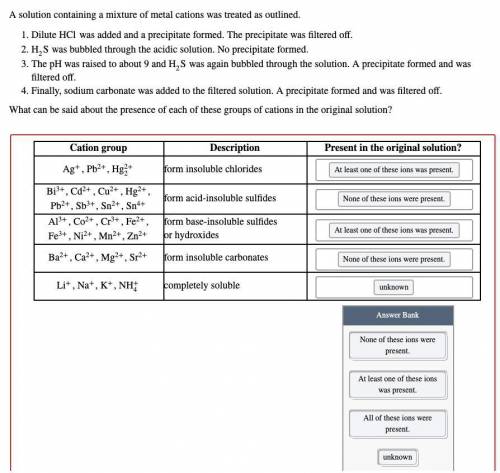
A solution containing a mixture of metal cations was treated as outlined.
Dilute HCl was added and a precipitate formed. The precipitate was filtered off.
H2S was bubbled through the acidic solution. No precipitate formed.
The pH was raised to about 9 and H2S was again bubbled through the solution. A precipitate formed and was filtered off.
Finally, sodium carbonate was added to the filtered solution. A precipitate formed and was filtered off.
What can be said about the presence of each of these groups of cations in the original solution?
Options: None of these ions were present
At least one of these ions was present
All of these ions were present
Unknown

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2

Chemistry, 23.06.2019 03:00
Air pressure is measured in pascals. for a professional american football game, the ball should be inflated to about 90,000 pascals. scientists studied the effects of air temperature on the pressure inside american footballs by taking these steps: 1. prepare 100 footballs. 2. measure each football's air pressure. 3. divide footballs into 10 groups. 4. place the groups in different lockers cooled to different air temperatures. 5. after 12 hours, remove the footballs from lockers. 6. measure each football's pressure again. 7. compare the new pressures to the starting pressures. what two terms best describe the variable "air pressure inside the football" in this experiment? independent, qualitative independent, quantitative dependent, qualitative dependent, quantitative
Answers: 3
You know the right answer?
A solution containing a mixture of metal cations was treated as outlined.
Dilute HCl was added and...
Questions




Mathematics, 11.11.2019 22:31





Mathematics, 11.11.2019 22:31






English, 11.11.2019 22:31

Mathematics, 11.11.2019 22:31

Chemistry, 11.11.2019 22:31

History, 11.11.2019 22:31


Chemistry, 11.11.2019 22:31



