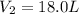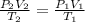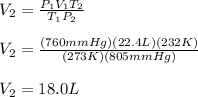Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1


Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2
You know the right answer?
1.00 mole of an ideal gas occupies a volume of 22.4 L at 0 C and 760 mmHg (STP). It is cooled to -41...
Questions


Social Studies, 02.03.2021 01:00


Arts, 02.03.2021 01:00




Mathematics, 02.03.2021 01:00

Mathematics, 02.03.2021 01:00




History, 02.03.2021 01:00

Biology, 02.03.2021 01:00



Computers and Technology, 02.03.2021 01:00

Mathematics, 02.03.2021 01:00