
Chemistry, 18.05.2021 21:10 marahsenno
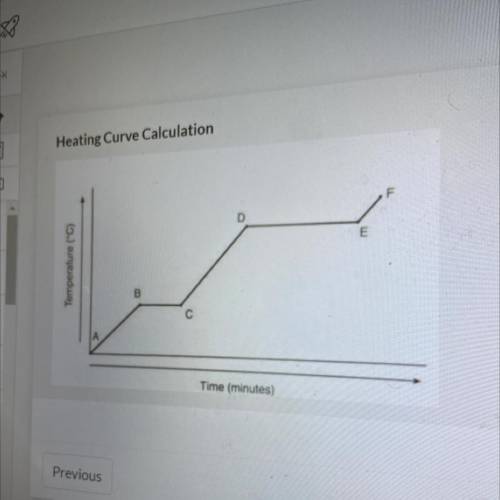
Referencing the figure to the left, how much energy in J would be
needed to change 59 g of ice at -22.75 °C into steam at 128.65 °C
Important numbers/equations:
q = Cpm: AT
Specific Heat of Water (liquid) = 4.18 J/go°C
Specific Heat of Water (solid) = 2.06 J/g•°C
Specific Heat of Water (gas) = 2.01 J/g °C
Molar Heat of Fusion of water: 6009 J/mol
Molar Heat of Vaporization for water: 40790 J/mol

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

You know the right answer?
Referencing the figure to the left, how much energy in J would be
needed to change 59 g of ice at -...
Questions

Mathematics, 25.08.2021 02:50




Mathematics, 25.08.2021 02:50

Chemistry, 25.08.2021 02:50


Business, 25.08.2021 02:50


History, 25.08.2021 02:50

Mathematics, 25.08.2021 02:50

English, 25.08.2021 02:50




Mathematics, 25.08.2021 02:50

Mathematics, 25.08.2021 02:50

History, 25.08.2021 02:50

Social Studies, 25.08.2021 02:50



