
Chemistry, 21.05.2021 01:00 walkinginmypurpose
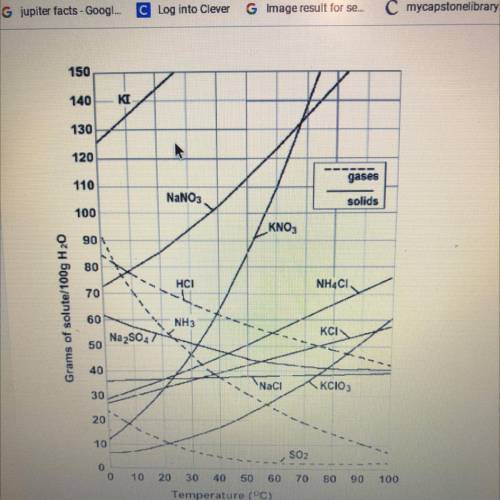
A saturated solution of Ammonium Chloride is dissolved in 100 g of water.
If the saturated solution is cooled from 50°C to 30°C, how many grams of
precipitate will be formed?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3

Chemistry, 22.06.2019 21:20
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: (s) (aq) (s) (aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of .
Answers: 2
You know the right answer?
A saturated solution of Ammonium Chloride is dissolved in 100 g of water.
If the saturated solution...
Questions



Law, 05.05.2020 16:44




Computers and Technology, 05.05.2020 16:44

Mathematics, 05.05.2020 16:44

Computers and Technology, 05.05.2020 16:44



Engineering, 05.05.2020 16:44

Computers and Technology, 05.05.2020 16:44

English, 05.05.2020 16:44

Mathematics, 05.05.2020 16:44

Mathematics, 05.05.2020 16:44

English, 05.05.2020 16:44

Computers and Technology, 05.05.2020 16:44





