
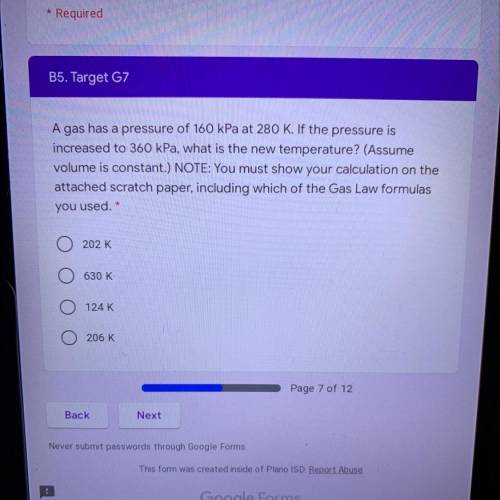
A gas has a pressure of 160 kPa at 280 K. If the pressure is
increased to 360 kPa, what is the new temperature? (Assume
volume is constant.) NOTE: You must show your calculation on the
attached scratch paper, including which of the Gas Law formulas
you used. *
A. 202 K
B. 630 K
C. 124 K
D. 206 K

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2


Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

You know the right answer?
A gas has a pressure of 160 kPa at 280 K. If the pressure is
increased to 360 kPa, what is the new...
Questions


Mathematics, 22.07.2019 00:32



Social Studies, 22.07.2019 00:32


Mathematics, 22.07.2019 00:32

Chemistry, 22.07.2019 00:32





Mathematics, 22.07.2019 00:32

History, 22.07.2019 00:32


Health, 22.07.2019 00:32

Mathematics, 22.07.2019 00:32



History, 22.07.2019 00:32



