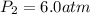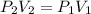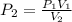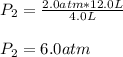Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Perform the following mathematical operations and report the answer to the appropriate number of significant figures 5.87998 + 3.100
Answers: 2


Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
You know the right answer?
An ideal gas at 2.0 atm pressure and 298 K temperature has a volume of 12.0 L. If the volume is decr...
Questions

Chemistry, 02.10.2020 19:01

English, 02.10.2020 19:01



Health, 02.10.2020 19:01

Mathematics, 02.10.2020 19:01




Mathematics, 02.10.2020 19:01

Mathematics, 02.10.2020 19:01

English, 02.10.2020 19:01

English, 02.10.2020 19:01

English, 02.10.2020 19:01

Biology, 02.10.2020 19:01

Business, 02.10.2020 19:01


Social Studies, 02.10.2020 19:01

History, 02.10.2020 19:01

Mathematics, 02.10.2020 19:01