Use the following balanced equation to answer the questions below.
...

Chemistry, 03.06.2021 08:10 missalawode28
Use the following balanced equation to answer the questions below.
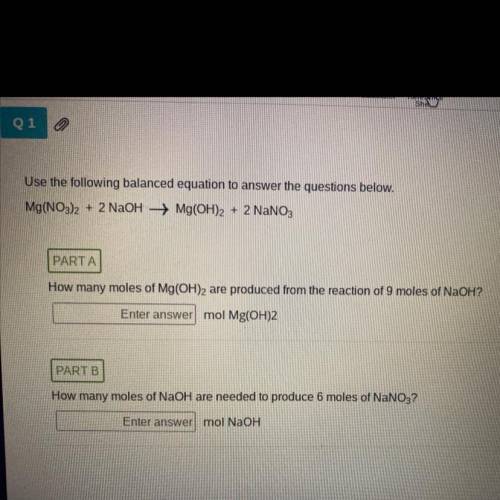

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3



Chemistry, 23.06.2019 10:00
The image shows the process of which is used in nuclear power plants. photo attached
Answers: 1
You know the right answer?
Questions

Health, 26.04.2021 22:30




Social Studies, 26.04.2021 22:30




Mathematics, 26.04.2021 22:30



Mathematics, 26.04.2021 22:30




Mathematics, 26.04.2021 22:30




Mathematics, 26.04.2021 22:30



