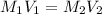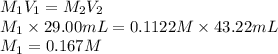Chemistry, 07.06.2021 15:30 biggiecheese93
A 29.00 mL sample of an H2SO4 solution of unknown concentration is titrated with a 0.1122 M KOH solution. A volume of 43.22 mL of KOH was required to reach the equivalence point. What is the concentration of the unknown H2SO4 solution? Express your answer using four significant figures.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

You know the right answer?
A 29.00 mL sample of an H2SO4 solution of unknown concentration is titrated with a 0.1122 M KOH solu...
Questions



Mathematics, 28.01.2020 11:31


Chemistry, 28.01.2020 11:31



Chemistry, 28.01.2020 11:31


English, 28.01.2020 11:31




English, 28.01.2020 11:31

English, 28.01.2020 11:31



Biology, 28.01.2020 11:31


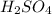 solution is 0.167 M.
solution is 0.167 M.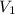 = 29.00 mL,
= 29.00 mL, 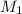 = ?
= ?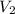 = 43.22 mL,
= 43.22 mL, 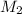 = 0.1122 M
= 0.1122 M