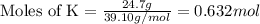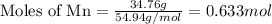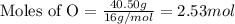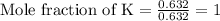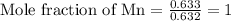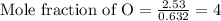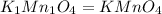Chemistry, 09.06.2021 20:40 supergraciepie
Determine the empirical formula of a compound having the following percent composition by mass: K: 24.74%; Mn: 34.76%; O: 40.50%

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3

Chemistry, 23.06.2019 04:50
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table.state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2
You know the right answer?
Determine the empirical formula of a compound having the following percent composition by mass: K: 2...
Questions




Biology, 27.11.2019 21:31






History, 27.11.2019 21:31

Mathematics, 27.11.2019 21:31


Arts, 27.11.2019 21:31



Geography, 27.11.2019 21:31

Mathematics, 27.11.2019 21:31


Chemistry, 27.11.2019 21:31

Mathematics, 27.11.2019 21:31

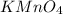
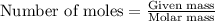 ......(1)
......(1)