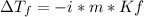Chemistry, 13.06.2021 14:00 VgCarlos3787
What is the expected freezing-point depression for a solution that contains 2.0 mol of KCI
(electrolyte) dissolved in 1.0 kg of water? (K,- -1.86°C/m.)
a.-7.44 °C
C. +7.44 °C
b.-4.77 °C
d. +4.7 °C

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

You know the right answer?
What is the expected freezing-point depression for a solution that contains 2.0 mol of KCI
(electro...
Questions

English, 31.03.2020 05:02

Mathematics, 31.03.2020 05:03

Mathematics, 31.03.2020 05:03

Mathematics, 31.03.2020 05:03




History, 31.03.2020 05:03






Mathematics, 31.03.2020 05:03

Mathematics, 31.03.2020 05:03

Mathematics, 31.03.2020 05:03

Biology, 31.03.2020 05:03



Engineering, 31.03.2020 05:03