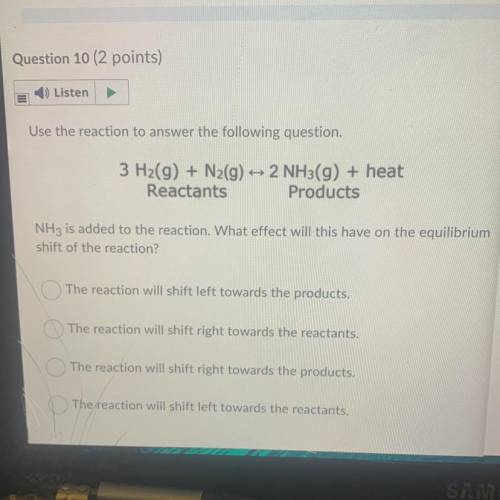
Use the reaction to answer the following question.
3 H2(g) + N2(g) + 2 NH3(g) + heat
Reactant...

Use the reaction to answer the following question.
3 H2(g) + N2(g) + 2 NH3(g) + heat
Reactants Products
NH3 is added to the reaction. What effect will this have on the equilibrium
shift of the reaction?
The reaction will shift left towards the products.
The reaction will shift right towards the reactants.
The reaction will shift right towards the products.
The reaction will shift left towards the reactants.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 22.06.2019 21:20
Juju.01) 5 geologic events a group of students designed an experiment in an ice rink to represent the solar system. the steps of the experiment are listed below. 5: geologic events 1. choose a student to represent the sun. the planets are represented by two tennis balls. 2. ask the student to hold the tennis balls in each palm and spin on the ice with arms stretched out. 3. ask the student to draw in the arms after about 10 spins. 4. observe the student's arms rotate faster when they are closer to the body. 05 geologic events enors) the experiment most likely demonstrates that (2 points) 07 discussion-based sessment/module planets exert gravitational force on the sun the sun exerts gravitational force on the planets 3.07 discussion-based ssessment speed of a planet depends on its distance from the sun new version available! (3.0.119) get it now submit 18.07: module exam description
Answers: 3

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1
You know the right answer?
Questions


Mathematics, 04.11.2020 21:20

Biology, 04.11.2020 21:20

Mathematics, 04.11.2020 21:20




English, 04.11.2020 21:20

Mathematics, 04.11.2020 21:20

Mathematics, 04.11.2020 21:20

Mathematics, 04.11.2020 21:20






Mathematics, 04.11.2020 21:20

Chemistry, 04.11.2020 21:20

Spanish, 04.11.2020 21:20



