
Chemistry, 30.06.2021 02:10 alesiabarrios6
Na3N decomposes to form sodium and nitrogen gas at STP. If 13.7 L of nitrogen is produced
how many moles of Na3N was used? (22.4 L = 1 mole of any gas)
2Na3N --> 6Na + N2

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2


Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
Na3N decomposes to form sodium and nitrogen gas at STP. If 13.7 L of nitrogen is produced
how many...
Questions

SAT, 28.01.2021 02:30

Mathematics, 28.01.2021 02:30

Mathematics, 28.01.2021 02:30

Social Studies, 28.01.2021 02:30


English, 28.01.2021 02:30

Social Studies, 28.01.2021 02:30








Chemistry, 28.01.2021 02:30

Mathematics, 28.01.2021 02:30


Mathematics, 28.01.2021 02:30


Mathematics, 28.01.2021 02:30

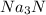 were used.
were used.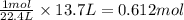
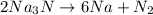
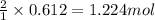 of
of 

