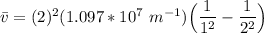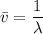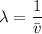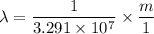Chemistry, 08.07.2021 15:50 lalkjlkeu2409
The energy levels of hydrogenlike one-electron ions of atomic number Z differ from those of hydrogen by a factor of Z^2. Predict the wavelength of the 2s--->1s transition in He+.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
You know the right answer?
The energy levels of hydrogenlike one-electron ions of atomic number Z differ from those of hydrogen...
Questions

Mathematics, 30.01.2021 01:00


Mathematics, 30.01.2021 01:00



Mathematics, 30.01.2021 01:00

Mathematics, 30.01.2021 01:00

English, 30.01.2021 01:00

Mathematics, 30.01.2021 01:00

Mathematics, 30.01.2021 01:00

Mathematics, 30.01.2021 01:00



Biology, 30.01.2021 01:00

Biology, 30.01.2021 01:00

Mathematics, 30.01.2021 01:00

English, 30.01.2021 01:00


Business, 30.01.2021 01:00

Mathematics, 30.01.2021 01:00

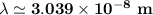
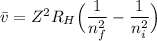
 = ???
= ???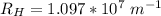
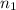 = 2
= 2