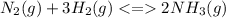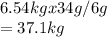Chemistry, 27.07.2021 02:00 dbzrules02
A reaction vessel for synthesizing ammonia by reacting nitrogen and hydrogen is charged with 6.54 kg of H2 and excess N2. A total of 30.4 kg of NH3 are produced. What is the percent yield of the reaction

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2
You know the right answer?
A reaction vessel for synthesizing ammonia by reacting nitrogen and hydrogen is charged with 6.54 kg...
Questions


French, 05.01.2021 09:50




Mathematics, 05.01.2021 09:50

SAT, 05.01.2021 09:50


Mathematics, 05.01.2021 09:50

Mathematics, 05.01.2021 09:50

Mathematics, 05.01.2021 14:00

Social Studies, 05.01.2021 14:00

Social Studies, 05.01.2021 14:00



History, 05.01.2021 14:00




Mathematics, 05.01.2021 14:00