4
D:
According to the phase diagram for H20, what happens to the phases of
water at 1 a...

4
D:
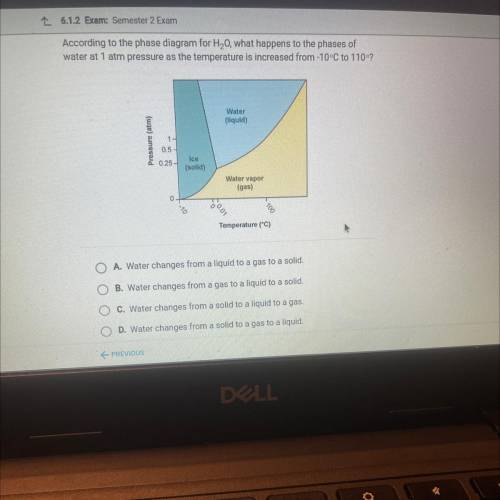
According to the phase diagram for H20, what happens to the phases of
water at 1 atm pressure as the temperature is increased from-10-C to 1102
Water
liquid
Pressure (atm)
0.5
025-
Ice
(solid)
Water vapor
(gas)
0
Temperature (°C)
O A. Water changes from a liquid to a gas to a solid
O B. Water changes from a gas to a liquid to a solid.
O C. Water changes from a solid to a liquid to a gas.
O D. Water changes from a solid to a gas to a liquid

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
You know the right answer?
Questions

Mathematics, 04.04.2020 05:52

Biology, 04.04.2020 05:52

Mathematics, 04.04.2020 05:52

Mathematics, 04.04.2020 05:52






Geography, 04.04.2020 05:52



Mathematics, 04.04.2020 05:53

Mathematics, 04.04.2020 05:53

Computers and Technology, 04.04.2020 05:53


Computers and Technology, 04.04.2020 05:53

Physics, 04.04.2020 05:53




