
Chemistry, 07.08.2021 01:00 radaishasmithoxngbj
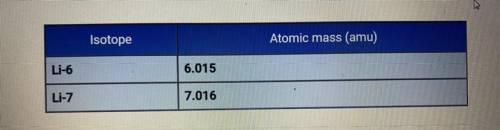
The average atomic mass of lithium is 6.94 amu. Based on the atomic
masses of the two isotopes of lithium, how do the relative abundances of the
isotopes compare?
A. Li-6 is much more abundant than Li-7.
B. They are about the same.
C. Li-7 is much more abundant than Li-6.
D. Li-7 is slightly more abundant than Li-6.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3


Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 23.06.2019 06:40
8. how much enthalpy/heat is transferred when 0.5113gof ammonia (nh3) reacts with excess oxygen according| to the following equation: 4nh3 +502 - 4n0+ 6h20ah = -905.4j
Answers: 1
You know the right answer?
The average atomic mass of lithium is 6.94 amu. Based on the atomic
masses of the two isotopes of l...
Questions




Mathematics, 14.03.2022 05:30

Mathematics, 14.03.2022 05:30

Mathematics, 14.03.2022 05:30


History, 14.03.2022 05:30






Mathematics, 14.03.2022 05:40


Spanish, 14.03.2022 05:40

Mathematics, 14.03.2022 05:40


English, 14.03.2022 05:40

Mathematics, 14.03.2022 05:40



