What is the freezing point, in °C,
of a 1.56 m aqueous solution
of K3PO4?
Note: K for...

Chemistry, 25.08.2021 20:10 kyahshayovvu24
What is the freezing point, in °C,
of a 1.56 m aqueous solution
of K3PO4?
Note: K for water is 1.86 °C/m
Enter rounded answer to three decimal points.
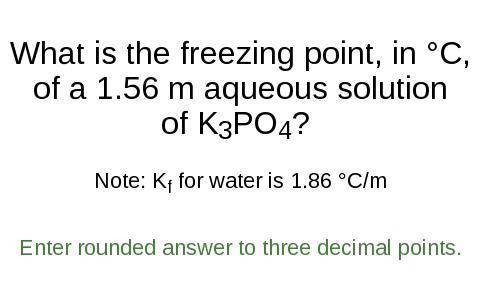

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1

Chemistry, 22.06.2019 07:30
The scheme below is from a series of reactions that are part of a synthesis of vitamin a. answer the following questions with reference to this scheme. (i) what is "reagent a"? (ii) draw a step-by-step mechanism which explains the formation of compound c from compound b (iii) which reagents would you use to form compound e from compounds c and d (reagents b and c)? for each reagent suggested above in (ii) explain the role of the reagent in the reaction to (iv) form compound e. you may wish to do this by drawing a mechanism. 1. addition of reagent a но reagent a 2. н,о" thо oh нон-с compound a. compound b. compound c .ch-оh 1. reagent b "сно 2. reagent c сh oh compound e. compound d.
Answers: 2

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3
You know the right answer?
Questions


Social Studies, 27.08.2019 20:10


Mathematics, 27.08.2019 20:10

English, 27.08.2019 20:10

Mathematics, 27.08.2019 20:10



History, 27.08.2019 20:10



English, 27.08.2019 20:10






Mathematics, 27.08.2019 20:10

Computers and Technology, 27.08.2019 20:10



