
Chemistry, 30.08.2021 04:20 barstr9146
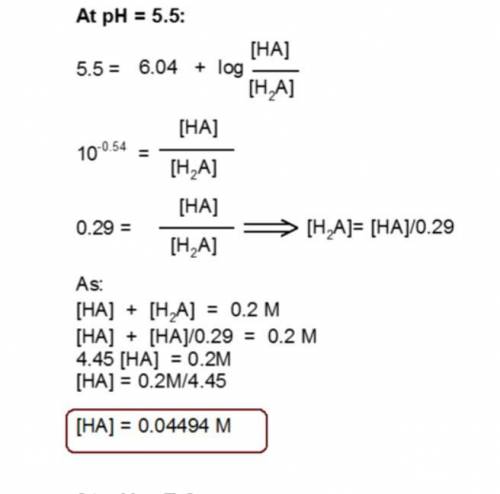
You have prepared a .2 M histidine solution. Calculate the molar concentration of isoelectric histidine at a) pH 5.5 and b) pH 7.0. A solution is provided, but can you do a step-by-step as I do not quite understand the math that went into this.


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1
You know the right answer?
You have prepared a .2 M histidine solution. Calculate the molar concentration of isoelectric histid...
Questions












Computers and Technology, 10.03.2020 09:01




English, 10.03.2020 09:01




English, 10.03.2020 09:01



