
Chemistry, 27.09.2021 01:00 mrsdeanwinchester18
Cho dung dịch Ba(OH)2 dư vào 50ml dung dịch X chứa các ion NH4+; SO42-; NO3- đun nóng thì có 11,65g kết tủa xuất hiện và có 4,48lít khí Y thoát ra. Nồng độ mỗi muối trong dung dịch X là
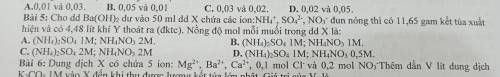

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

You know the right answer?
Cho dung dịch Ba(OH)2 dư vào 50ml dung dịch X chứa các ion NH4+; SO42-; NO3- đun nóng thì có 11,65g...
Questions

History, 22.07.2019 04:00



History, 22.07.2019 04:00

Mathematics, 22.07.2019 04:00



Biology, 22.07.2019 04:00


Biology, 22.07.2019 04:00


Business, 22.07.2019 04:00


Mathematics, 22.07.2019 04:00


Social Studies, 22.07.2019 04:00



Mathematics, 22.07.2019 04:00



