
Chemistry, 03.10.2021 01:00 memoryofdale
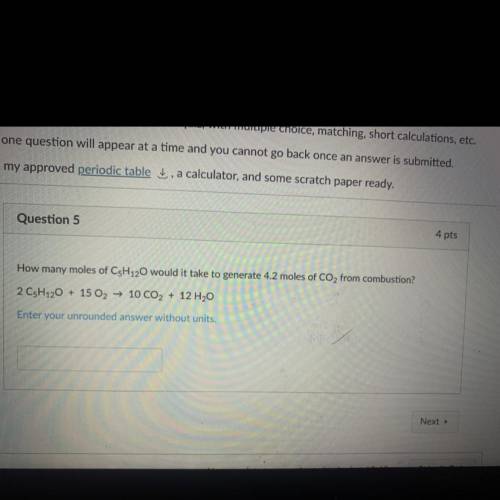
How many moles of C5H120 would it take to generate 4.2 moles of CO2 from combustion?
2 CsH120 + 15 O2 → 10 CO2 + 12 H2O
Enter your unrounded answer without units.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 23.06.2019 07:00
How does science use models to gain a better understanding of concepts?
Answers: 1
You know the right answer?
How many moles of C5H120 would it take to generate 4.2 moles of CO2 from combustion?
2 CsH120 + 15...
Questions

Advanced Placement (AP), 22.04.2021 18:00

Mathematics, 22.04.2021 18:00


Mathematics, 22.04.2021 18:00

English, 22.04.2021 18:00





Mathematics, 22.04.2021 18:00

Arts, 22.04.2021 18:00




Mathematics, 22.04.2021 18:00

Mathematics, 22.04.2021 18:00


Mathematics, 22.04.2021 18:00




