
Chemistry, 04.10.2021 14:00 daniel1480
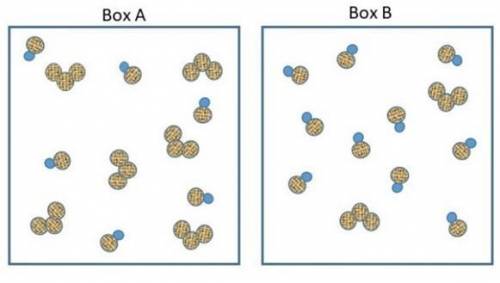
The two pictures shown below represent starting conditions for the following reaction: O3 (g) + NO(g) → O2 (g) + NO2 (g) with a rate law: Rate = k (O3 )(NO)
Which flask will react faster than the other? Determine how much faster the fast one reacts compared to the slow one? Explain your answers in terms of molecular collisions.
How do I find the answer to this? My thinking is that box a will react more since there's equal amounts of reactants but box b will react faster since there's so much NO to collide with the O3 molecules. Thanks.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1

Chemistry, 23.06.2019 01:00
Animals that reproduce sexually either do it through external or internal fertilization. read the following statement and decide if it is true or false. birds reproduce through external reproduction which is because the female will then be able to protect the egg.
Answers: 1
You know the right answer?
The two pictures shown below represent starting conditions for the following reaction: O3 (g) + NO(g...
Questions






Mathematics, 05.12.2019 21:31


Arts, 05.12.2019 21:31



English, 05.12.2019 21:31

English, 05.12.2019 21:31


Social Studies, 05.12.2019 21:31



Mathematics, 05.12.2019 21:31

Spanish, 05.12.2019 21:31





