
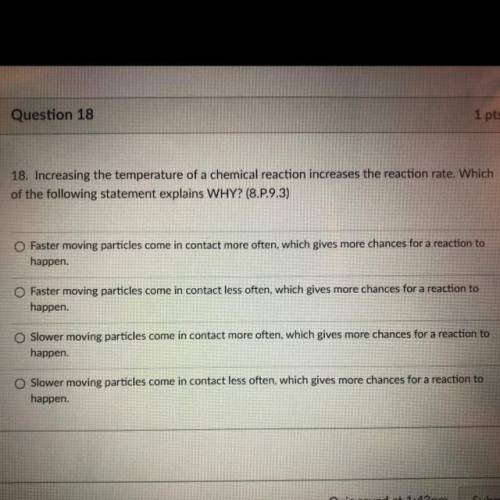
Chemistry, 06.10.2021 21:20 davidswafforddd478
18. Increasing the temperature of a chemical reaction increases the reaction rate. Which
of the following statement explains WHY? (8.P.9.3)
O Faster moving particles come in contact more often, which gives more chances for a reaction to
happen.
Faster moving particles come in contact less often, which gives more chances for a reaction to
happen
Slower moving particles come in contact more often, which gives more chances for a reaction to
happen.
Slower moving particles come in contact less often, which gives more chances for a reaction to
happen.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

You know the right answer?
18. Increasing the temperature of a chemical reaction increases the reaction rate. Which
of the fo...
Questions


Social Studies, 06.10.2021 03:10

Mathematics, 06.10.2021 03:10

Social Studies, 06.10.2021 03:10

English, 06.10.2021 03:10



Mathematics, 06.10.2021 03:10



Physics, 06.10.2021 03:10


Mathematics, 06.10.2021 03:10

History, 06.10.2021 03:10

Mathematics, 06.10.2021 03:10

Social Studies, 06.10.2021 03:10

Business, 06.10.2021 03:10

SAT, 06.10.2021 03:10


English, 06.10.2021 03:10



