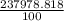Chemistry, 04.11.2021 09:30 arushiverma555
Uranium has three common isotopes: U-238 (99.2745%), U-235 (0.7200%), U-234 (0.0055%). Calculate the average atomic mass of uranium. (Round to two decimal places)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Acar tire has a pressure of 2.38 atm at 15.2 c. if the pressure inside reached 4.08 atm, the tire will explode. how hot would the tire have to get for this to happen? report the temperature in degrees celsius.
Answers: 2

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1

Chemistry, 23.06.2019 05:50
What are the coefficients to balance the following equation? ba+br=babr2
Answers: 1
You know the right answer?
Uranium has three common isotopes: U-238 (99.2745%), U-235 (0.7200%), U-234 (0.0055%). Calculate the...
Questions



Mathematics, 28.06.2019 14:00

Mathematics, 28.06.2019 14:00

Social Studies, 28.06.2019 14:00

Physics, 28.06.2019 14:00

Mathematics, 28.06.2019 14:00

History, 28.06.2019 14:00


Mathematics, 28.06.2019 14:00

Mathematics, 28.06.2019 14:00

History, 28.06.2019 14:00


Social Studies, 28.06.2019 14:00


Social Studies, 28.06.2019 14:00


Spanish, 28.06.2019 14:00


Mathematics, 28.06.2019 14:00