
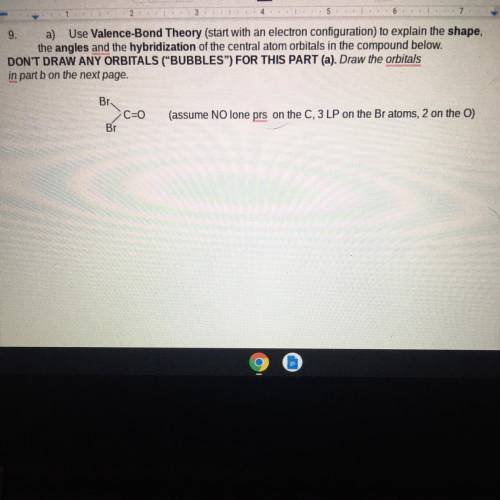
A. Photo Below B. Now draw those atomic orbitals you obtained in part a and then overlap them to form the C-Br bonds (you only have to draw one C-Br Bond) and bonds of the C=O. Label the atomic orbitals of all atoms and also label the resulting bonds as o symbol or pi symbol.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1


Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2
You know the right answer?
A. Photo Below
B. Now draw those atomic orbitals you obtained in part a and then overlap them to f...
Questions



Computers and Technology, 21.01.2020 18:31







Arts, 21.01.2020 18:31


Mathematics, 21.01.2020 18:31

History, 21.01.2020 18:31


German, 21.01.2020 18:31







