Question 9 of 32
The reaction below is at equilibrium. What would happen if more carbon were
...

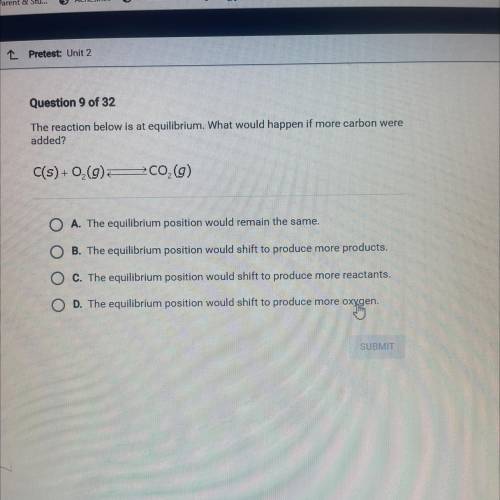
Question 9 of 32
The reaction below is at equilibrium. What would happen if more carbon were
added?
C(s) +0,0 200,0)
A. The equilibrium position would remain the same.
B The equilibrium position would shift to produce more products.
The equilibrium position would shift to produce more reactants.
The equilibrium position would shift to produce more oxygen
SUNT

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2


Chemistry, 22.06.2019 22:00
8) warming your hands by a fire is an example if which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 1
You know the right answer?
Questions

Chemistry, 22.08.2019 10:50

English, 22.08.2019 10:50

Social Studies, 22.08.2019 10:50


Mathematics, 22.08.2019 10:50

Biology, 22.08.2019 10:50





History, 22.08.2019 10:50

Chemistry, 22.08.2019 10:50

Physics, 22.08.2019 10:50

Mathematics, 22.08.2019 10:50

History, 22.08.2019 10:50

Arts, 22.08.2019 10:50


Physics, 22.08.2019 10:50

Computers and Technology, 22.08.2019 10:50

Social Studies, 22.08.2019 10:50



