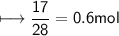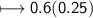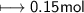Chemistry, 16.01.2022 05:50 ashleyvalles16
Calculate the number of moles of SO2 required to produce 17 g of CO in the following chemical equation: 5 C + 2 SO2 → CS2 + 4 CO

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:20
Determine which intermolecular forces are the dominant (strongest) forces for a pure sample of each of the following molecules by placing the molecules into the correct bins. drag the appropriate molecular formula to their respective bins.
Answers: 3

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1
You know the right answer?
Calculate the number of moles of SO2 required to produce 17 g of CO in the following chemical equati...
Questions


Mathematics, 16.04.2021 18:00


English, 16.04.2021 18:00

Mathematics, 16.04.2021 18:00

Mathematics, 16.04.2021 18:00



Advanced Placement (AP), 16.04.2021 18:00

Mathematics, 16.04.2021 18:00


Mathematics, 16.04.2021 18:00

Mathematics, 16.04.2021 18:00

History, 16.04.2021 18:00

Mathematics, 16.04.2021 18:00

English, 16.04.2021 18:00

Mathematics, 16.04.2021 18:00