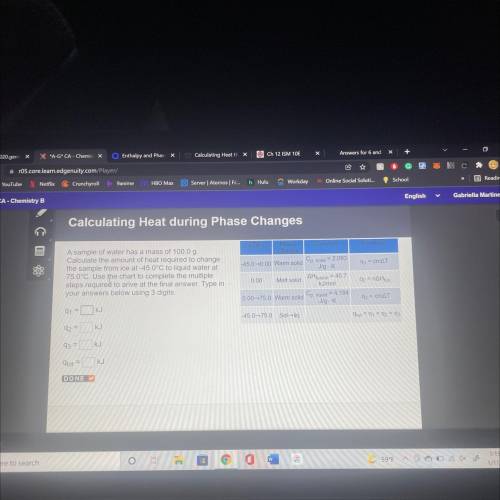
A sample of water has a mass of 100.00
Calculate the amount of heat required to change
the s...

Chemistry, 19.01.2022 14:00 somethingar183
A sample of water has a mass of 100.00
Calculate the amount of heat required to change
the sample from ice at 450°C to liquid water at
78.0°C. Use the chart to complete the multiple
steps required to arrive at the final answer. Type in your answers below using 3 digits.
Q1= KJ
Q2= KJ
Q3= KJ
q(tot)= KJ

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1
You know the right answer?
Questions

Mathematics, 23.06.2019 03:00

Mathematics, 23.06.2019 03:00


Mathematics, 23.06.2019 03:00


History, 23.06.2019 03:00

Biology, 23.06.2019 03:00



Chemistry, 23.06.2019 03:00

Mathematics, 23.06.2019 03:00



Biology, 23.06.2019 03:00






Health, 23.06.2019 03:00



