
Chemistry, 31.01.2022 07:10 harleypage308
Please help!
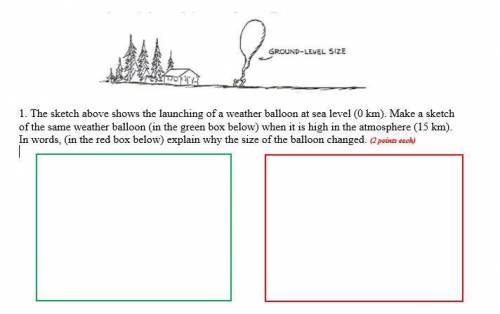
1. The sketch above shows the launching of a weather balloon at sea level (0 km). Make a sketch of the same weather balloon (in the green box below) when it is high in the atmosphere (15 km). In words, (in the red box below) explain why the size of the balloon changed
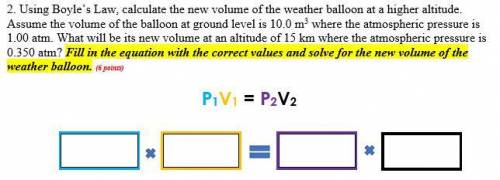
2. Using Boyle’s Law, calculate the new volume of the weather balloon at a higher altitude. Assume the volume of the balloon at ground level is 10.0 m3 where the atmospheric pressure is 1.00 atm. What will be its new volume at an altitude of 15 km where the atmospheric pressure is 0.350 atm? Fill in the equation with the correct values and solve for the new volume of the weather balloon.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2
You know the right answer?
Please help!
1. The sketch above shows the launching of a weather balloon at sea level (0 km). Mak...
Questions







Mathematics, 21.04.2020 19:10


Mathematics, 21.04.2020 19:10


Biology, 21.04.2020 19:10

Mathematics, 21.04.2020 19:10



Mathematics, 21.04.2020 19:10

English, 21.04.2020 19:10

Computers and Technology, 21.04.2020 19:10



Physics, 21.04.2020 19:10



