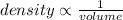As a person squeezes and applies pressure to the outside of a balloon, air particles inside a) will occupy less volume causing a decrease in temperature. b) will occupy less volume causing an increase in the density of the particles. c) will collide more often causing a decrease in the density of the particles. d) will slow down causing the volume of the balloon to shrink.

Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3

Chemistry, 23.06.2019 07:30
To separate a mixture of hard candies nd marbles the most efficient method would be
Answers: 3
You know the right answer?
As a person squeezes and applies pressure to the outside of a balloon, air particles inside a) will...
Questions

Mathematics, 27.10.2021 01:00

Chemistry, 27.10.2021 01:00

Mathematics, 27.10.2021 01:00

Mathematics, 27.10.2021 01:00

Mathematics, 27.10.2021 01:00


Mathematics, 27.10.2021 01:00

Mathematics, 27.10.2021 01:00

Mathematics, 27.10.2021 01:00

History, 27.10.2021 01:00




Chemistry, 27.10.2021 01:00

Mathematics, 27.10.2021 01:00

History, 27.10.2021 01:00


History, 27.10.2021 01:00


Mathematics, 27.10.2021 01:00

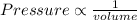 volume is inversely proportional to the density,
volume is inversely proportional to the density,