
Chemistry, 02.08.2019 22:30 rubimachuca1020
In the reaction so2 (g) + h2o (l) ↔ h2so3 (aq), with k = 2.1 × 10–3, the concentration of so2 is 0.35 m, and the concentration of h2so3 is 0.23 m. this reaction a. is in equilibrium b. must shift to the reactants to be in equilibrium c. must shift to the products to be in equilibrium d. must have the pressure increased to reach equilibrium e. none of the above

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2

You know the right answer?
In the reaction so2 (g) + h2o (l) ↔ h2so3 (aq), with k = 2.1 × 10–3, the concentration of so2 is 0.3...
Questions

Computers and Technology, 18.01.2021 21:20

Mathematics, 18.01.2021 21:20










History, 18.01.2021 21:20



Physics, 18.01.2021 21:20





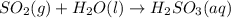
![Q=\frac{[H_2SO_3(aq)]}{[SO_2(g)]}](/tpl/images/0163/3606/a76c3.png)
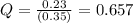
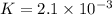
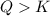 that means product > reactant. So, the reaction is reactant favored.
that means product > reactant. So, the reaction is reactant favored.
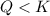 that means reactant > product. So, the reaction is product favored.
that means reactant > product. So, the reaction is product favored.
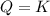 that means product = reactant. So, the reaction is in equilibrium.
that means product = reactant. So, the reaction is in equilibrium.

