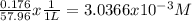Chemistry, 30.07.2019 01:30 Alexandragurule18
Calculate the external pressure that must be applied to seawater, 1.14 m total ion concentration at 10 degrees c if the maximum concentration allowed in the product water is 176 mg/l. assume that all the dissolved salts in the product water is sodium chloride. so, i know that i need to subtract the ion concentrations before using the pi=mrt formula, but i can't figure out how to convert mg/l into molarity. !

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
You know the right answer?
Calculate the external pressure that must be applied to seawater, 1.14 m total ion concentration at...
Questions











Mathematics, 03.11.2020 18:00

Mathematics, 03.11.2020 18:00




Biology, 03.11.2020 18:00




History, 03.11.2020 18:00