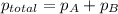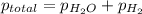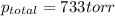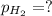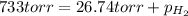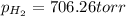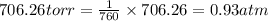Chemistry, 29.07.2019 01:30 dasdsadsafdhgifsdu
Zinc reacts with aqueous sulfuric acid to form hydrogen gas: zn(s) + h2so4(aq) → znso4(aq) + h2(g) in an experiment, 201 ml of wet h2 is collected over water at 27°c and a barometric pressure of 733 torr. the vapor pressure of water at 27°c is 26.74 torr. the partial pressure of hydrogen in this experiment is atm.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 21.06.2019 23:00
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1
You know the right answer?
Zinc reacts with aqueous sulfuric acid to form hydrogen gas: zn(s) + h2so4(aq) → znso4(aq) + h2(g)...
Questions



Chemistry, 04.08.2020 21:01



Chemistry, 04.08.2020 21:01

History, 04.08.2020 21:01







History, 04.08.2020 21:01




History, 04.08.2020 21:01


Mathematics, 04.08.2020 21:01