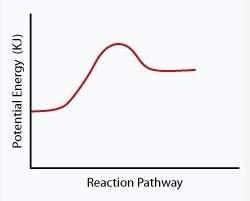
The following phase diagram shows how a catalyst affected the rate of a reaction.
which...

Chemistry, 26.09.2019 08:10 conniehodson
The following phase diagram shows how a catalyst affected the rate of a reaction.
which statement below best describes how the catalyst affected the reaction shown in this diagram?
a. the catalyst decreased the activation energy of the reactants.
b. the catalyst increased the activation energy of the reactants.
c. the catalyst increased activation energy of the products.
d. the catalyst decreased activation energy of the products.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1

Chemistry, 23.06.2019 03:30
The semi-conductors on the periodic table are classified as
Answers: 1
You know the right answer?
Questions

History, 21.09.2020 02:01

Mathematics, 21.09.2020 02:01

English, 21.09.2020 02:01


Mathematics, 21.09.2020 02:01


English, 21.09.2020 02:01



Mathematics, 21.09.2020 02:01

Advanced Placement (AP), 21.09.2020 02:01

History, 21.09.2020 02:01

World Languages, 21.09.2020 02:01



Social Studies, 21.09.2020 02:01



Mathematics, 21.09.2020 02:01



