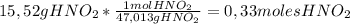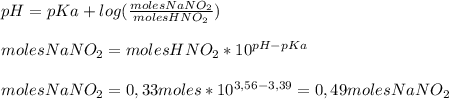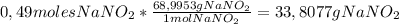Chemistry, 25.07.2019 06:30 raishagibson
A1.00 l solution contains 15.52 g of nitrous acid, hno2. what mass of sodium nitrite, nano2, should be added to it to make a buffer with a ph of 3.56? ka (hno2) = 4.0 × 10–4.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
A1.00 l solution contains 15.52 g of nitrous acid, hno2. what mass of sodium nitrite, nano2, should...
Questions


Physics, 02.08.2019 08:30

Mathematics, 02.08.2019 08:30

Biology, 02.08.2019 08:30

History, 02.08.2019 08:30

Mathematics, 02.08.2019 08:30


English, 02.08.2019 08:30

Mathematics, 02.08.2019 08:30






Social Studies, 02.08.2019 08:30